Rotterdam, The Netherlands – October 12, 2021 – Quantib, a market leader in artificial intelligence solutions for precision diagnosis, announced today that they are launching the newest update of the Quantib® Prostate solution, Quantib® Prostate 1.3. This software for prostate MRI reading support has a class IIb CE marking under the new MDR laws and FDA clearance for clinical use in the United States. Therefore being available for both the European and the USA market.
“Quantib has been an incredibly responsive and innovative company. Their constructive use of feedback and suggestions is part of contributing to a team effort toward product development and solutions”, said Dan Sperling, MD. “I am pleased with the frequency with which they bring new features and product enhancements each quarter”.
About Quantib® Prostate
Quantib® Prostate, leveraging the power of deep learning algorithms, advances the MRI prostate reporting workflow and is accessible directly from the radiologist’s reading station. Moreover, the solution comes with a suite of tools to improve reporting quality and speed: including AI-based volumetry and PSA density calculation, precise registration and movement correction, one-click segmentation of lesion candidates, classification, PI-RADS scoring support and standardized reporting to facilitate easy and comprehensive communication of results. The upgrade includes:
- Kinetic curve representation per ROI and of the full prostate gland
- Visual PI-RADS v.2.1 scoring support including ROI marking
- Compatibility with multiple ultrasound systems to import 3D gland and lesion segmentations
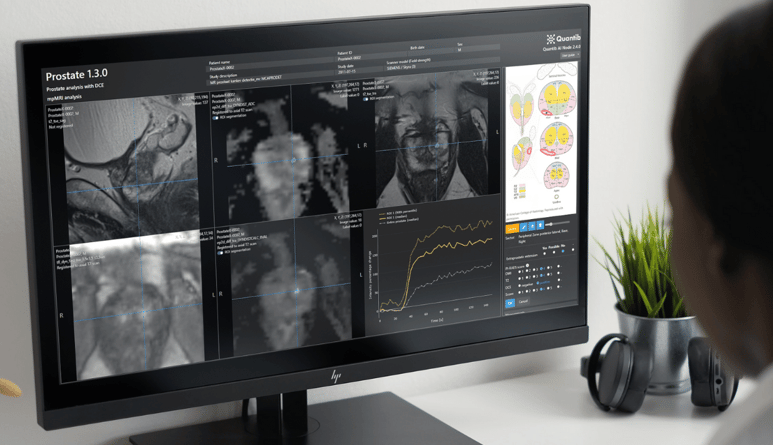
“The most important thing for us is listening to our clients and incorporating their feedback to improve our products taking their wishes into account” Quirine, Quantib’s COO said. “We have worked very hard to improve compatibility and further optimize the AI-powered reports according to our customers’ needs and we look forward to rolling out this update in the coming weeks”.
About Quantib
Quantib’s AI radiology solutions enable faster and more accurate diagnosis by quickly identifying abnormalities, supporting adequate and timely patient care. Using state-of-the-art artificial intelligence techniques, Quantib’s software advances the diagnostic pathway by providing FDA-cleared and CE marked solutions related to neurodegeneration and prostate cancer. All deployed through our Quantib AI Node platform enabling seamless workflow integration and rapid regulatory clearance.