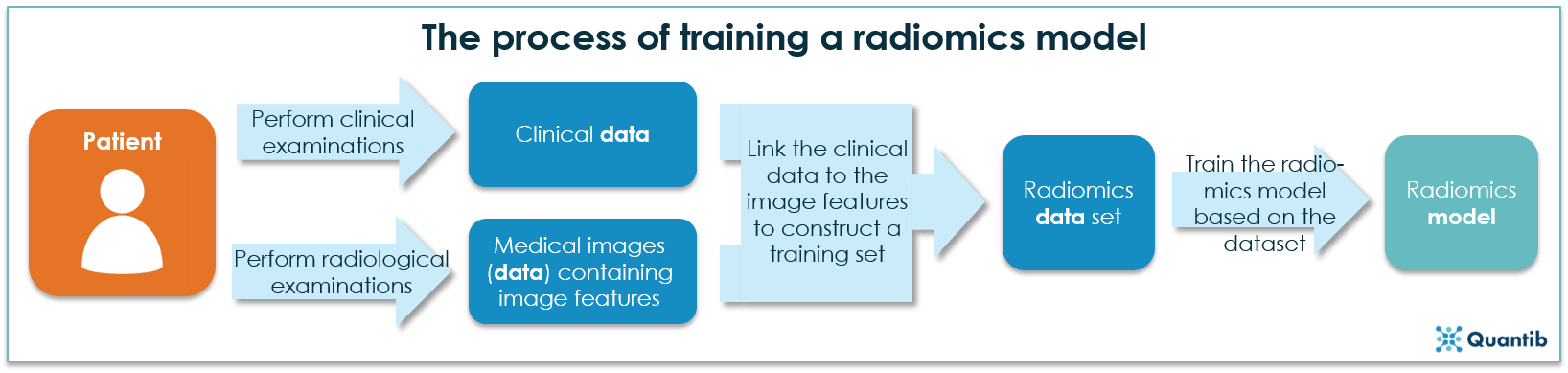
The field of radiomics covers a range of methods aimed at the extraction of quantitative features from medical images and linking these features to pathological data. Developing algorithms to perform radiomics starts with collecting large amounts of medical images and clinical data. Subsequently, certain features (shapes, tissue texture, etc.) are extracted from the medical images, which, thereafter, can be linked with the clinical data, resulting in a radiomics training set. By presenting this combined training set to an artificial intelligence algorithm, the computer builds an understanding of the patterns that exist in how the medical images link to the clinical data, i.e. a radiomics model is developed. See the figure below for a schematic overview.
Within the field of neuro-oncology, radiomics offers several opportunities to improve our understanding of brain tumors. As explained by Zhou et al. in a recent review paper, the deployment of radiomics to automatically analyze and identify gliomas, offers a wide range of possibilities. These include standardized tumor staging and more differentiated diagnosis, as well as refined monitoring of treatment response, e.g. following radiation treatment.1
Radiomics, machine learning, and deep learning: a promising combination
Radiomics is often paired with machine learning, a process of teaching an algorithm to recognize a given trait or event by going through large amounts of data and “learning” from the information contained therein. Deep learning is a specific subset of machine learning that deploys deep neural networks to learn how to perform a specific task. Deep learning algorithms may be able to exploit image features which are not straightforward for humans to interpret, but which may be characteristic for a specific disease.
Machine learning can, for example, be a great tool to develop an algorithm for standardized assessment of tumor grading based on currently known features. Deep learning offers valuable options for algorithm development when it comes to determining new image features of brain tumors which can contribute to more differentiated diagnosis (e.g. by recognizing tumor subtypes on an image).
In the remainder of this blog post, we will address 3 promising opportunities of radiomics in the field of Brain Tumor Diagnosis and Prognosis.
1. Radiomics enables (more) standardized tumor grading
MRI based brain tumor grading can be a challenging task, since medical images may simultaneously contain image features typical of both low grade and high grade neoplasms, while tumors may also contain both low and high grade components complicating diagnosis. In addition, imaging results may be ambiguous due to overlap of tumor tissue and normal tissue.2,3 Radiomics may assist by facilitating standardized and automated brain tumor staging as the following recent investigations demonstrate:
Using both conventional and perfusion MRI scans of 102 patients suffering from brain tumors, Zacharaki et al. investigated the use of machine learning based algorithms to discriminate between high and low grade neoplasms (WHO grades III/IV and II, respectively), with a resulting accuracy of 88%. Zacharaki furthermore developed an algorithm which could distinguish between metastases and gliomas, with an accuracy of 85%.4
Khalwaldeh et al. applied convolutional neural networks, typical for deep learning, to classify MRI scans into 3 different categories: 1) images displaying healthy tissue, 2) images displaying low grade gliomas (astrocytoma and oligodendroglioma grade II) and 3) images displaying high grade gliomas (glioblastoma, astrocytoma III and oligodendroglioma III). An overall accuracy of over 90% was obtained, based on 587 cases.5
2. Radiomics opens up the path for differentiated diagnosis based on genotyping, supporting clinical decision making
Using radiomics to genotype solely based on MRI features was shown by Yiming et al. Diffuse gliomas often carry a mutation of the p53 gene, which can, at present, only be detected from tumor tissue obtained by performing a craniotomy. Yiming selected a set of radiomic image features such as the shape and size of the tumors, features quantifying intra-tumoral heterogeneity and features quantitatively describing the voxel distribution. Accuracy of the research was determined by deducing the optimal cut-off area using an area under the curve analysis (using a specificity vs. sensitivity graph). The accuracy measured 76% to 90%, indicating that a machine learning approach can deliver a robust method to determine a genotype solely using MR scans.8
In a study led by Dr. Marion Smits (Erasmus MC, Rotterdam, funded by the Dutch Cancer Society), an algorithm has been developed to recognize the visual difference between glioma with 1p/19q codeletion (oligodendroglioma) and glioma without (astrocytoma). A first version of the algorithm, based on MRI scans and genetic markup of 80 patients diagnosed with gliomas, reached an accuracy of 79-95%, compared to 67-77% for (neuro)radiologists who were asked to analyze the same set of MRI scans. Currently, the algorithm is being fine tuned by expanding the set of training images and validated in an external dataset.9
Intra-tumor heterogeneity? Let radiomics pave the way
Medical images of brain tumors can contain heterogeneity internally, creating an additional field of research for algorithm development. Similar to how radiomics is deployed to differentiate between the brain tumor types, features can be determined to deduce the different molecular subtypes of e.g. glioblastoma. Sub-regional image analysis can shed light on the different molecular activities within a single tumor, opening up possibilities for targeted treatments.1
3. Radiomics allows for more accurate treatment tracking by differentiating between tumor recurrence and tissue necrosis
Tumors and radiation therapy-induced necrotic tissue can appear similar on follow-up scans (i.e. increased contrast enhancement and high SI on T2-weighted images), making it hard to distinguish between the two entities. Computer scientists proved that computational methods may offer a robust solution.10,11
Hu et al. showed that a machine learning approach using multiparametric MRI features for differentiation between tumor recurrence and necrotic tissue, is able to achieve sensitivity and specificity numbers of approximately 90%. It should be noted, however, that the algorithm was only based on scans of 31 patients, likely obtained using a single scanner. For the development of robust algorithms, it is necessary to increase the dataset used for training significantly - both in size and diversity.12
Other recent studies extracted image features from multi-parametric MRI, deducting subtle differences between recurring tumors (for primary tumors as well as metastases) and radiation necrosis. Researchers used a method based on regression analysis to determine an importance score for each of the 119 image features used. Features of the type “Histogram of gradient orientations” (these are features aimed to detect objects by counting the occurrence of gradients) were identified as most important for distinguishing between tumor tissue and necrosis tissue.13
To conclude on radiomics
The radiomics examples described in this article only form the tip of the iceberg when it comes to the challenges and possibilities offered by radiomics for brain tumor diagnosis and prognosis.
Next to the promising opportunities radiomics provides for application in the clinic, great value can be added to scientific research as well. Different types of brain tumors can be investigated and understood in greater detail. Further possibilities include radiomics based research investigating new cancer treatments and longitudinal radiomics. It is obvious that the use of radiomics in the future can play a significant role in patient welfare by making diagnostic procedures less invasive.
It can be concluded that great potential lies in applying radiomics to neuro-oncology. It is important to note, however, that most results obtained to date need to be refined before they can be applied in the clinic.
Curious to learn more about how Quantib is deploying radiomics to improve brain tumor diagnosis? Subscribe to our newsletter and hear about the latest developments of our radiomics research project!
Bibliography
- Zhou, M. et al. Radiomics in Brain Tumor: Image Assessment, Quantitative Feature Descriptors, and Machine-Learning Approaches. Am. J. Neuroradiol. 39, 208–216 (2018).
- Krabbe, K. et al. MR diffusion imaging of human intracranial tumours. Neuroradiology 39, 483–9 (1997).
- Aronen, H. J. et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191, 41–51 (1994).
- Zacharaki, E. I. et al. Classification of brain tumor type and grade using MRI texture and shape in a machine learning scheme. Magn. Reson. Med. 62, 1609–18 (2009).
- Khawaldeh, S., Pervaiz, U., Rafiq, A. & Alkhawaldeh, R. Noninvasive Grading of Glioma Tumor Using Magnetic Resonance Imaging with Convolutional Neural Networks. Appl. Sci. 8, 27 (2017).
- Smits, M. & van den Bent, M. J. Imaging Correlates of Adult Glioma Genotypes. Radiology 284, 316–331 (2017).
- Aldape, K. et al. Glioma Through the Looking GLASS: Molecular Evolution of Diffuse Gliomas and the Glioma Longitudinal AnalySiS Consortium. Neuro. Oncol. (2018). doi:10.1093/neuonc/noy020
- Li, Y. et al. MRI features predict p53 status in lower-grade gliomas via a machine-learning approach. NeuroImage. Clin. 17, 306–311 (2018).
- van der Voort, S. R. et al. Radiogenomic classification of the 1p/19q status in presumed low-grade gliomas. in 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017) 638–641 (IEEE, 2017). doi:10.1109/ISBI.2017.7950601
- Verma, N., Cowperthwaite, M. C., Burnett, M. G. & Markey, M. K. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro. Oncol. 15, 515–534 (2013).
- Six, O. R. (2018, 5, 31). Email exchange with Deserno, W.M.L.L.G., MD, MSc, PhD.
- Hu, X., Wong, K. K., Young, G. S., Guo, L. & Wong, S. T. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. J. Magn. Reson. Imaging 33, 296–305 (2011).
- Pallavi, T. et al. Texture Descriptors to distinguish Radiation Necrosis from Recurrent Brain Tumors on multi-parametric MRI. Proc. SPIE--the Int. Soc. Opt. Eng. 9035, 90352B (2014).

